-
Product Name
Anti-GRP78 BiP (3B4) Mouse antibody
- Documents
-
Description
GRP78 BiP (3B4) Mouse monoclonal antibody
-
Tested applications
WB, IHC-P
-
Species reactivity
Human
-
Isotype
Mouse IgG1
-
Preparation
Antigen: Purified recombinant fragment of human HSPA5 expressed in E. Coli.
-
Clonality
Monoclonal
-
Formulation
Ascitic fluid containing 0.03% sodium azide.
-
Storage instructions
Store at 4°C short term. Store at -20°C long term. Avoid freeze / thaw cycle.
-
Applications
WB: 1/500 - 1/2000
IHC: 1/200 - 1/1000
ELISA: 1/10000
-
Validations
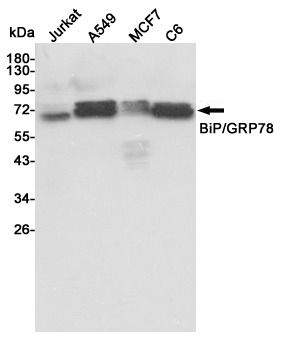
Western blot analysis of extracts from Jurkat,A549,MCF7 and C6 cell lysates using BiP/GRP78 mouse mAb (1:1000 diluted).Predicted band size:72KDa.Observed band size:72KDa.
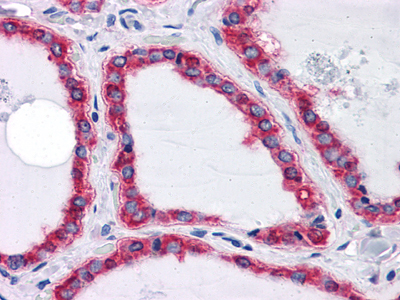
Immunohistochemical analysis of paraffin-embedded human Thyroid tissues using HSPA5 mouse mAb
-
Background
Swiss-Prot Acc.P11021.When Chinese hamster K12 cells are starved of glucose, the synthesis of several proteins, called glucose-regulated proteins (GRPs), is markedly increased. Hendershot et al. (1994) (PubMed 8020977) pointed out that one of these, GRP78 (HSPA5), also referred to as 'immunoglobulin heavy chain-binding protein' (BiP), is a member of the heat-shock protein-70 (HSP70) family and is involved in the folding and assembly of proteins in the endoplasmic reticulum (ER). Because so many ER proteins interact transiently with GRP78, it may play a key role in monitoring protein transport through the cell.Probably plays a role in facilitating the assembly of multimeric protein complexes inside the ER.The HSP70 proteins are ubiquitous molecular chaparones that are found in all organisms and tissue types. Like other members of the HSP70 family, BiP is a peptide-binding ATPase that is able to differentiate native proteins from unfolded polypeptides. BiP does not bind to fully folded and assembled proteins, except in the presence of other co-chaparones. BiP is involved in a number of key mechanisms and pathways including polypeptide translocation across the endoplasmic reticulum, folding, assembly, transport of secreted or membrane proteins, and the regulation of calcium homeostasis. Although BiP is relatively abundant, marked increases in BiP occur where there is an accumulation of unfolded polypeptides. For this reason, BiP has been identified as a marker for various disease states that are associated with secretory and transmembrane protein misfolding.
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
