-
Product Name
Human tPA recombinant protein
- Documents
-
Description
Tissue plasminogen activator (abbreviated tPA or PLAT), is traditionally viewed as a simple serine protease whose main function is to convert plasminogen into biologically active plasmin. As a protease, tPA plays a crucial role in regulating blood fibrinolysis, in maintaining the homeostasis of extracellular matrix and in modulating the post-translational activation of growth factors. tPA is synthesized and secreted as a single chain polypeptide precursor which is cleaved in turn by plasmin. Proteolytic cleavage at the C-terminal side of Arg275 generates the enzyme composed of two subunits, designated as α and β chains which are held together by a single disulfide bond. Unlike the other members of the chymotrypsin family, tPA has one particular distinction in that the catalytic efficiency of the single-chain enzyme is only slightly lower than that of the proteolytically cleaved form and is therefore not a true zymogen. tPA is found not only in the blood, where its primary function is as a thrombolytic enzyme, but also in the central nervous system (CNS). It participats in a number of physiological and pathological events in the CNS, as well as the role of neuroserpin as the natural regulator of tPA's activity in these processes. Increased or decreased activity of tPA leads to hyperfibrinolysis or hypofibrinolysis, respectively. In addition, as a cytokine, tPA plays a pivotal role in the pathogenesis of renal interstitial fibrosis through diverse mechanisms. Thus, as a fibrogenic cytokine, it promotes the progression of kidney diseases.
-
Protein name
Tissue-type plasminogen activator
-
Protein short names
T-PA; DKFZP686I03148; PLAT; TPA; D8ERTD2E; MGC18508; AW212668; AU020998
-
Uniprot ID
P00750
-
Gene Name
PLAT
-
Source/Expression Host
Human Cells
-
Expression Plasmid/cDNA
The ? chain (Ile 311-Pro 562) of mature human tPA (NP_000921.1) was obtained after cleavage of the N-terminal human IgG1 Fc region from the purified chimera.
-
Protein Species
Human
-
Molecular weight
The recombinant human tPA ? chain consists of 252 amino acids and has a predicted molecular mass of 28 kDa. As a result of glycosylation, the apparent molecular mass of rh tPA? is approximately 38 kDa in SDS-PAGE under reducing conditions.
-
Purity
> 95 % as determined by SDS-PAGE
-
Validations
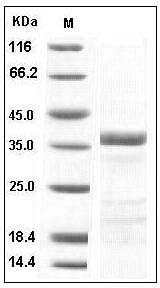
Human tPA / PLAT Protein SDS-PAGE
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
