-
Product Name
HSPE1 Polyclonal Antibody
- Documents
-
Description
Polyclonal antibody to HSPE1
-
Tested applications
WB, IHC, IF
-
Species reactivity
Human, Mouse, Rat
-
Alternative names
HSPE1 antibody; CPN10 antibody; EPF antibody; GROES antibody; HSP10 antibody; 10 kDa heat shock protein, mitochondrial antibody
-
Isotype
Rabbit IgG
-
Preparation
Antigen: Recombinant fusion protein containing a sequence corresponding to amino acids 1-102 of human HSPE1 (NP_002148.1).
-
Clonality
Polyclonal
-
Formulation
PBS with 0.02% sodium azide, 50% glycerol, pH7.3.
-
Storage instructions
Store at -20℃. Avoid freeze / thaw cycles.
-
Applications
WB 1:500 - 1:2000
IHC 1:50 - 1:200
IF 1:50 - 1:200 -
Validations
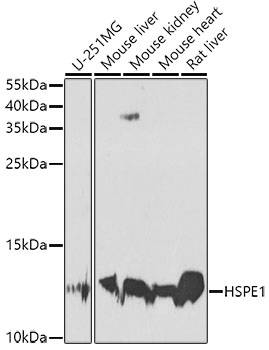
Western blot - HSPE1 Polyclonal Antibody
Western blot analysis of extracts of various cell lines, using HSPE1 antibody at 1:1000 dilution.Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) at 1:10000 dilution.Lysates/proteins: 25ug per lane.Blocking buffer: 3% nonfat dry milk in TBST.Detection: ECL Basic Kit .Exposure time: 90s.
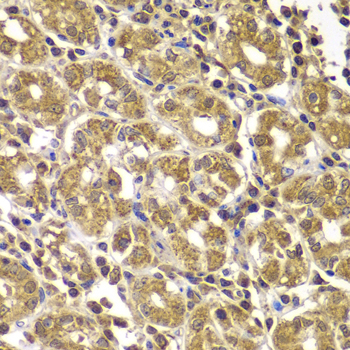
Immunohistochemistry - HSPE1 Polyclonal Antibody
Immunohistochemistry of paraffin-embedded human gastric using HSPE1 antibody at dilution of 1:100 (40x lens).
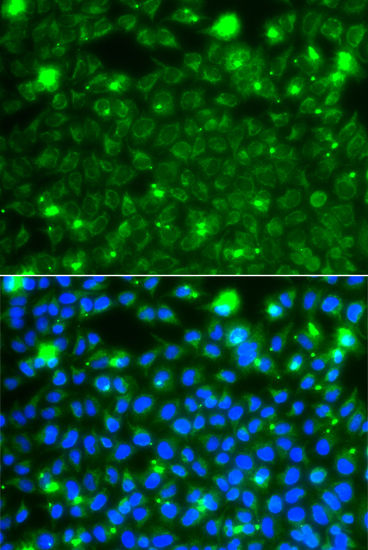
Immunofluorescence - HSPE1 Polyclonal Antibody
Immunofluorescence analysis of A549 cells using HSPE1 antibody . Blue: DAPI for nuclear staining.
-
Background
Co-chaperonin implicated in mitochondrial protein import and macromolecular assembly. Together with Hsp60, facilitates the correct folding of imported proteins. May also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix. The functional units of these chaperonins consist of heptameric rings of the large subunit Hsp60, which function as a back-to-back double ring. In a cyclic reaction, Hsp60 ring complexes bind one unfolded substrate protein per ring, followed by the binding of ATP and association with 2 heptameric rings of the co-chaperonin Hsp10. This leads to sequestration of the substrate protein in the inner cavity of Hsp60 where, for a certain period of time, it can fold undisturbed by other cell components. Synchronous hydrolysis of ATP in all Hsp60 subunits results in the dissociation of the chaperonin rings and the release of ADP and the folded substrate protein (Probable).
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
