-
Product Name
AlaRS antibody
- Documents
-
Description
AlaRS Rabbit Polyclonal antibody. Positive IHC detected in human liver tissue. Positive IF detected in HepG2 cells. Positive IP detected in HepG2 cells. Positive WB detected in HepG2 cells, HeLa cells, Jurkat cells. Observed molecular weight by Western-blot: 107 kDa
-
Tested applications
ELISA, WB, IHC, IP, IF
-
Species reactivity
Human,Mouse,Rat; other species not tested.
-
Alternative names
AARS antibody; Alanine tRNA ligase antibody; alanyl tRNA synthetase antibody; AlaRS antibody
-
Isotype
Rabbit IgG
-
Preparation
This antibody was obtained by immunization of AlaRS recombinant protein (Accession Number: NM_001605). Purification method: Antigen affinity purified.
-
Clonality
Polyclonal
-
Formulation
PBS with 0.02% sodium azide and 50% glycerol pH 7.3.
-
Storage instructions
Store at -20℃. DO NOT ALIQUOT
-
Applications
Recommended Dilution:
WB: 1:200-1:1000
IP: 1:200-1:2000
IHC: 1:20-1:200
IF: 1:20-1:200
-
Validations
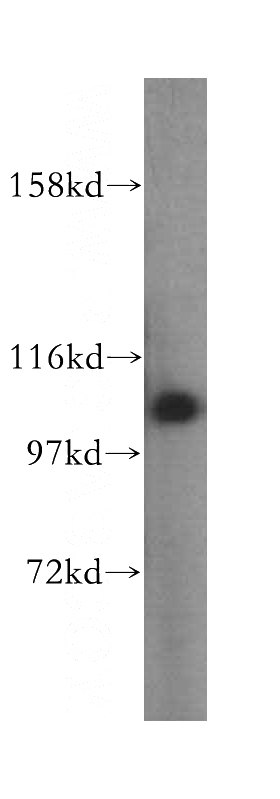
HepG2 cells were subjected to SDS PAGE followed by western blot with Catalog No:107953(AARS antibody) at dilution of 1:400
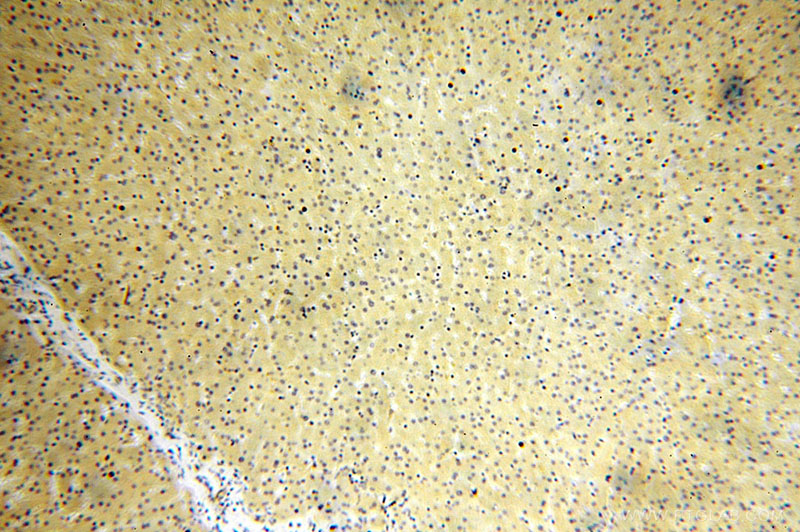
Immunohistochemical of paraffin-embedded human liver using Catalog No:107953(AARS antibody) at dilution of 1:100 (under 10x lens)
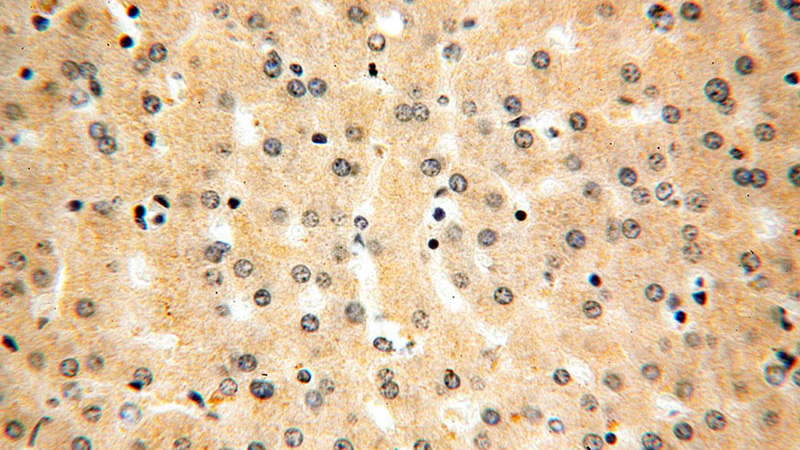
Immunohistochemical of paraffin-embedded human liver using Catalog No:107953(AARS antibody) at dilution of 1:100 (under 40x lens)
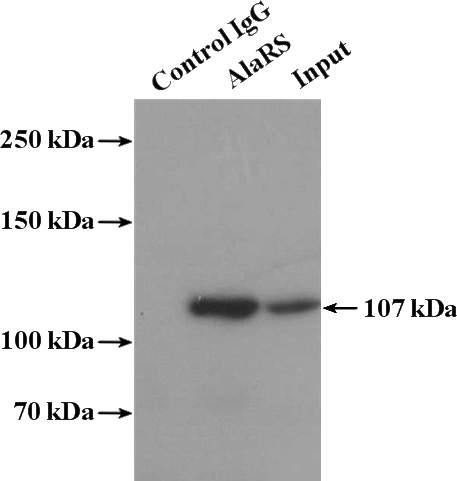
IP Result of anti-AARS (IP:Catalog No:107953, 4ug; Detection:Catalog No:107953 1:500) with HepG2 cells lysate 2400ug.
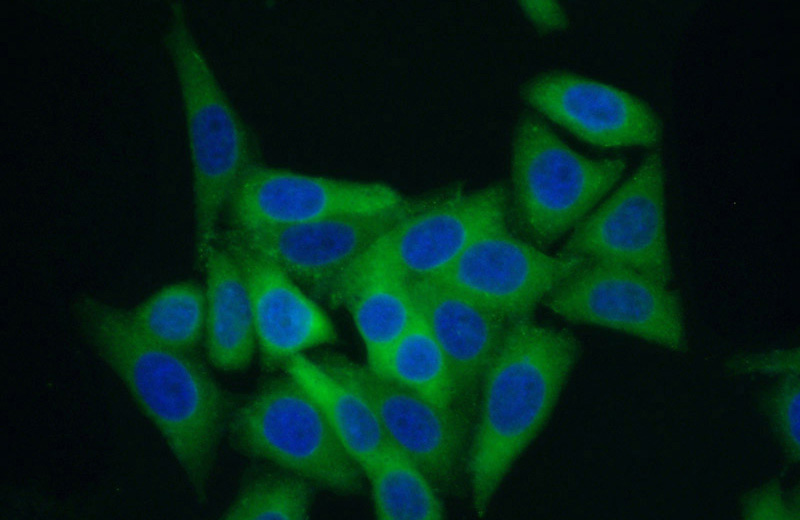
Immunofluorescent analysis of (10% Formaldehyde) fixed HepG2 cells using Catalog No:107953(AARS Antibody) at dilution of 1:50 and Alexa Fluor 488-congugated AffiniPure Goat Anti-Rabbit IgG(H+L)
-
Background
AARS(alanyl-tRNA synthetase) is also named as AlaRS(alanine tRNA ligase 1, cytoplasmic), renal carcinoma antigen NY-REN-42 and belongs to the class-II aminoacyl-tRNA synthetase family. It can interpret the RNA code and attach specific aminoacids to the tRNAs that contain the cognate trinucleotide anticodons. AARS consists of three domains: the N-terminal catalytic domain, the editing domain and the C-terminal C-Ala domain. The editing domain removes incorrectly charged amino acids, while the C-Ala domain, along with tRNA(Ala), serves as a bridge to cooperatively bring together the editing and aminoacylation centers thus stimulating deacylation of misacylated tRNAs(PMID:19661429).
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
