Cell-penetrating peptides (CPPs) are short peptides that facilitate cellular intake/uptake of various molecular equipment (from nanosize particles to small chemical molecules and large fragments of DNA). The "cargo" is associated with the peptides either through chemical linkage via covalent bonds or through non-covalent interactions. The function of the CPPs are to deliver the cargo into cells, a process that commonly occurs through endocytosis with the cargo delivered to delivery vectors for use in research and medicine.
CPPs typically have an amino acid composition that either contains a high relative abundance of positively charged amino acids such as lysine or arginine or has sequences that contain an alternating pattern of polar/charged amino acids and non-polar, hydrophobic amino acids. These two types of structures are referred to as polycationic or amphipathic, respectively. A third class of CPPs are the hydrophobic peptides, containing only apolar residues, with low net charge or have hydrophobic amino acid groups that are crucial for cellular uptake.
Table 1 provides a list of the most structurally and functionally characterized CPPs, the majority of which are currently in preclinical or clinical development.
| CPP name | Sequence | Origin | Class |
| HIV-1 TAT protein,TAT48-60 | GRKKRRQRRRPPQ | HIV-1 TAT protein | Cationic |
| HIV-1 TAT protein,TAT49-57 | RKKRRQRRR | HIV-1 TAT protein | Cationic |
| HIV-1 tat Protein (47-57) | YGRKKRRQRRR | HIV-1 TAT protein | Cationic |
| Penetratin,pAntp(43-58) | RQIKIWFQNRRMKWKK | AntennapediaDrosophilamelanogaster | Cationic |
| Polyarginines | Rn | Chemically synthesized | Cationic |
| DPV1047 | VKRGLKLRHVRPRVTRMDV | Chemically synthesized | Cationic |
| MPG | GALFLGFLGAAGSTMGAWSQPKKKRKV | HIV glycoprotein 41/ SV40 T antigen NLS | Amphipathic |
| Pep-1 | KETWWETWWTEWSQPKKKRKV | Tryptophan-richcluster/SV40 T antigen NLS | Amphipathic |
| pVEC | LLIILRRRIRKQAHAHSK | Vascular endothelial Cadherin | Amphipathic |
| ARF(1-22) | MVRRFLVTLRIRRACGPPRVRV | p14ARF protein | Amphipathic |
| BPrPr(1-28) | MVKSKIGSWILVLFVAMWSDVGLCKKRP | N terminus of unprocessed bovine prion protein | Amphipathic |
| MAP | KLALKLALKALKAALKLA | Chemically synthesized | Amphipathic |
| Transportan | GWTLNSAGYLLGKINLKALAALAKKIL | Chimeric galanin–Mastoparan | Amphipathic |
| p28 | LSTAADMQGVVTDGMASGLDKDYLKPDD | Azurin | Amphipathic |
| VT5 | DPKGDPKGVTVTVTVTVTGKGDPKPD | Chemically synthesized | Amphipathic |
| Bac 7 (Bac 1-24) | RRIRPRPPRLPRPRPRPLPFPRPG | Bactenecin family of antimicrobial peptides | Amphipathic |
| C105Y | CSIPPEVKFNKPFVYLI | a1-Antitrypsin | Hydrophobic |
| PFVYLI | PFVYLI | Derived from synthetic C105Y | Hydrophobic |
| Pep-7 | SDLWEMMMVSLACQY | CHL8 peptide phage Clone | Hydrophobic |
Cellular Uptake Mechanisms of CPPs
Although the mechanisms for cellular internalization of CPPs have been the subject of intense investigation, the pathways involved in this process have not been fully clarified. The difficulties encountered in the comprehension of the cellular uptake of these peptides are mostly ascribed to the differing physicochemical properties, size, and concentration of the diverse CPPs and/or CPP–cargo conjugates. These features can, indeed, have significant impact on the efficiency of cellular entry. Nonetheless, it has become clear that a single CPP can exploit different routes to enter the cell and that these routes may occasionally operate concomitantly, depending on the context of the experimental conditions. These entry routes are broadly divided into two groups: energy-independent direct penetration of the plasma membrane and energy dependent endocytosis. While direct translocation across the cell membrane occurs in some cases, mainly at high concentrations of the peptide, it is generally accepted that most CPPs and CPP–cargo conjugates enter cells by endocytosis.
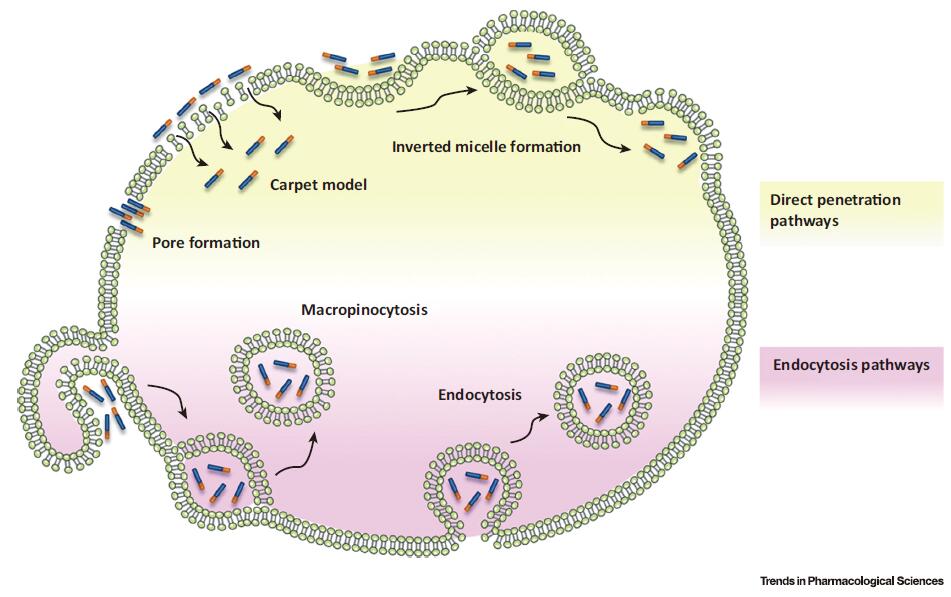
Fig.1. Schematic Representation of Proposed Mechanisms for Cell-Penetrating Peptide (CPP) Internalization. The diagram illustrates that the involved pathways can be divided into two groups: direct penetration of plasma membrane (yellow) and endocytic pathways (purple). The first type of process involves several energy independent models including membrane insertion of CPPs through pore formation and membrane destabilization through the carpet-like model or inverted micelle formation. Endocytic internalization of CPPs is an energy-dependent process that comprises macropinocytosis and endocytosis.
Clinical Applications of CPPs
The significant achievements in the preclinical evaluation of various CPP-derived peptide therapeutics, during the past decades, have revealed a remarkable potential for clinical application. Thus, several pharmaceutical companies have undertaken the clinical development of CPPs for local and systemic administration of various therapeutic molecules (Figure 2 and Table 2).
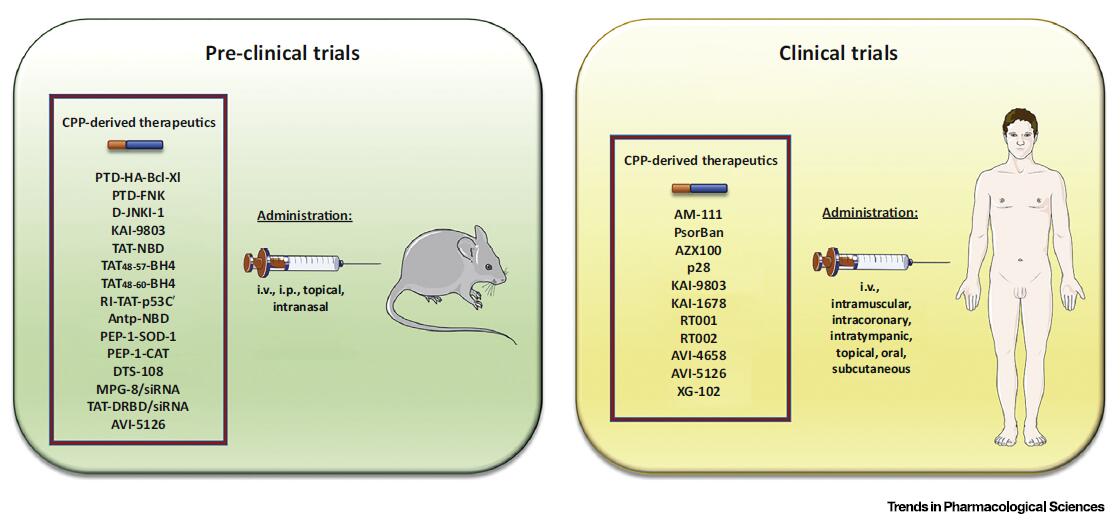
Fig. 2. Schematic Representation of Preclinical and Clinical Evaluations of Some Cell-Penetrating Peptide (CPP)-Derived Therapeutics. Numerous studies have been performed to investigate the therapeutic applications of various CPPs both in animal models of various diseases and in humans. Administration of CPP-derived therapeutics can be undertaken through relatively noninvasive administration routes, such as intravenous (i.v.), intraperitoneal (i.p.), intranasal, topical, intramuscular, per os, intracoronary, intratympanic, and subcutaneous.
Table 2. Examples of CPP-Conjugated Therapeutics Under Clinical Development
| Pharmaceutical organization | Compound | CPP-cargo | Therapeutic use |
| Auris Medical | AM-111 | TAT-JBD20(D-JNKI-1) | Hearing loss |
| CellGate, Inc. | PsorBan | R7-cyclosporin A | Psoriasis |
| Capstone Therapeutics | AZX100 | PTD4-HSP20Phosphopeptide | Scar prevention/reduction |
| CDG Therapeutics, Inc. | p28 | p28 | Cancer |
| KAI Pharmaceuticals | KAI-9803 | TAT–dPKC inhibitor | Myocardial infarction |
| KAI-1678 | TAT-ePKC inhibitor | Pain: postherpeticneuralgia, spinal cordinjury, postoperative | |
| RevanceTherapeutics, Inc. | RT001 | MTS-botulinumtoxin A | Lateral canthal lines, Crow's feetFacial wrinkles |
| RT002 | TransMTS1-botulinum toxin A | Glabellar lines | |
| Sarepta Therapeutics | AVI-4658 | N/A | Duchenne muscularDystrophy |
| AVI-5126 | (R-Ahx-R)4–PMO | Cardiovascular diseaseCoronary artery bypass | |
| Xigen SA | XG-102 | TAT-JBD20(D-JNKI-1) | Inflammation |
| Intraocular inflammationand pain |
Souce: https://doi.org/10.1016/j.tips.2017.01.003 2018-05-15
