-
Product Name
ERVW-1 Polyclonal Antibody
- Documents
-
Description
Polyclonal antibody to ERVW-1
-
Tested applications
WB
-
Species reactivity
Human, Mouse
-
Alternative names
ERVW-1 antibody; ENV antibody; ENVW antibody; ERVWE1 antibody; HERV-7q antibody; HERV-W-ENV antibody; HERV7Q antibody; HERVW antibody; HERVWENV antibody; syncytin-1 antibody
-
Isotype
Rabbit IgG
-
Preparation
Antigen: A synthetic peptide corresponding to a sequence within amino acids 1-100 of human ERVW-1 (NP_001124397.1).
-
Clonality
Polyclonal
-
Formulation
PBS with 0.02% sodium azide, 50% glycerol, pH7.3.
-
Storage instructions
Store at -20℃. Avoid freeze / thaw cycles.
-
Applications
WB 1:500 - 1:2000
-
Validations
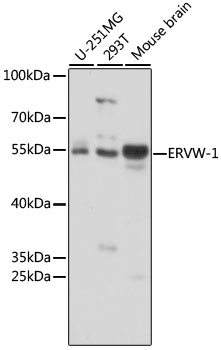
Western blot - ERVW-1 Polyclonal Antibody
Western blot analysis of extracts of various cell lines, using ERVW-1 antibody at 1:1000 dilution.Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) at 1:10000 dilution.Lysates/proteins: 25ug per lane.Blocking buffer: 3% nonfat dry milk in TBST.Detection: ECL Basic Kit .Exposure time: 3s.
-
Background
This endogenous retroviral envelope protein has retained its original fusogenic properties and participates in trophoblast fusion and the formation of a syncytium during placenta morphogenesis. May induce fusion through binding of SLC1A4 and SLC1A5.; Endogenous envelope proteins may have kept, lost or modified their original function during evolution. Retroviral envelope proteins mediate receptor recognition and membrane fusion during early infection. The surface protein (SU) mediates receptor recognition, while the transmembrane protein (TM) acts as a class I viral fusion protein. The protein may have at least 3 conformational states: pre-fusion native state, pre-hairpin intermediate state, and post-fusion hairpin state. During viral and target cell membrane fusion, the coiled coil regions (heptad repeats) assume a trimer-of-hairpins structure, positioning the fusion peptide in close proximity to the C-terminal region of the ectodomain. The formation of this structure appears to drive apposition and subsequent fusion of membranes.
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
