-
Product Name
Bevacizumab (Synonyms: PF-06439535 [WHO-DD]; USP MAB 002, MONOCLONAL IGG1 [USP-RS])
- Documents
-
Description
Anti-VEGFA Antibody (Bevacizumab)
-
Tested applications
ELISA
-
Species reactivity
Human VEGFA
-
Alternative names
PF-06439535 [WHO-DD] antibody; USP MAB 002, MONOCLONAL IGG1 [USP-RS] antibody
-
Isotype
IgG1
-
Preparation
Recombinant expression and purified from CHO cells.
-
Clonality
Monoclonal
-
Formulation
0.1 M Pro-Ac, 20 mM Arg, pH 5.0
-
Storage instructions
-80°C for 2 years under sterile conditions; -20°C for 1 year under sterile conditions; Avoid repeated freeze-thaw cycles.
-
Validations
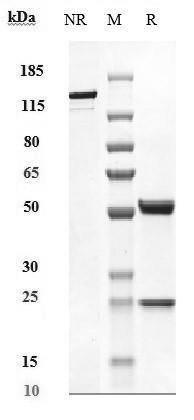
SDS-PAGE
Anti-VEGF Antibody (BioMab patent anti-VEGF) on SDS-PAGE under reducing (R) condition. The purity of the protein is greater than 95%.
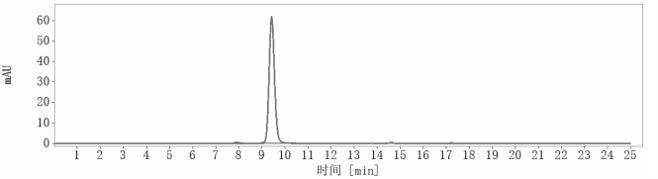
SEC-HPLC
The purity of Anti-VEGF Antibody (BioMab patent anti-VEGF) is 97.8%, determined by SEC-HPLC.
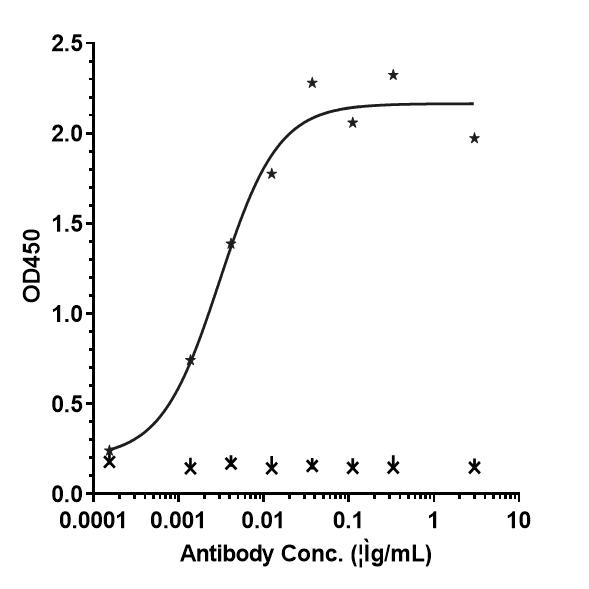
Bioactivity: ELISA
Immobilized human VEGF165 His at 2 ug/mL can bind Anti-VEGF Antibody (BioMab patent anti-VEGF), EC50=0.003018 ug/mL.
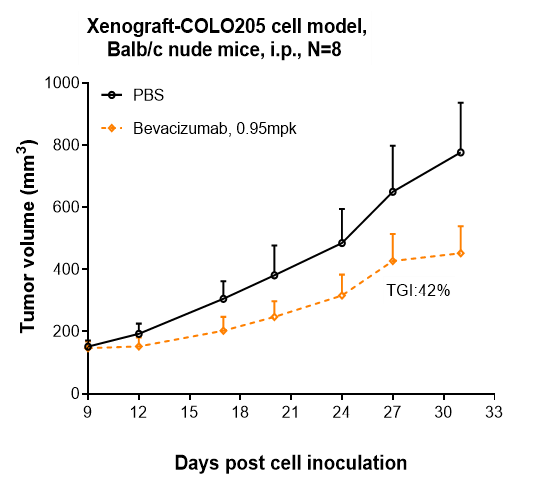
Research in vivo
Bevacizumab inhibited the tumor growth of COLO205 on balb/c nude mice. The result showed significant anti-tumor effects, with an tumor inhibition rate (TGI) of 42.0% at 0.95 mpk at D31.
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
