-
Product Name
Anti-STUB1 Rabbit antibody
- Documents
-
Description
STUB1 Rabbit monoclonal antibody
-
Tested applications
WB, ICC/IF, IP
-
Species reactivity
Human, Mouse, Rat
-
Alternative names
CHIP; UBOX1; SCAR16; HSPABP2; NY-CO-7; SDCCAG7 antibody
-
Isotype
Rabbit IgG
-
Preparation
Antigen: A synthetic peptide of human STUB1
-
Clonality
Monoclonal
-
Formulation
Supplied in 50nM Tris-Glycine(pH 7.4), 0.15M Nacl, 40%Glycerol, 0.01% sodium azide and 0.05% BSA.
-
Storage instructions
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze / thaw cycle.
-
Applications
1:2000-1:10000
1:50-1:200
1:20-1:50
-
Validations
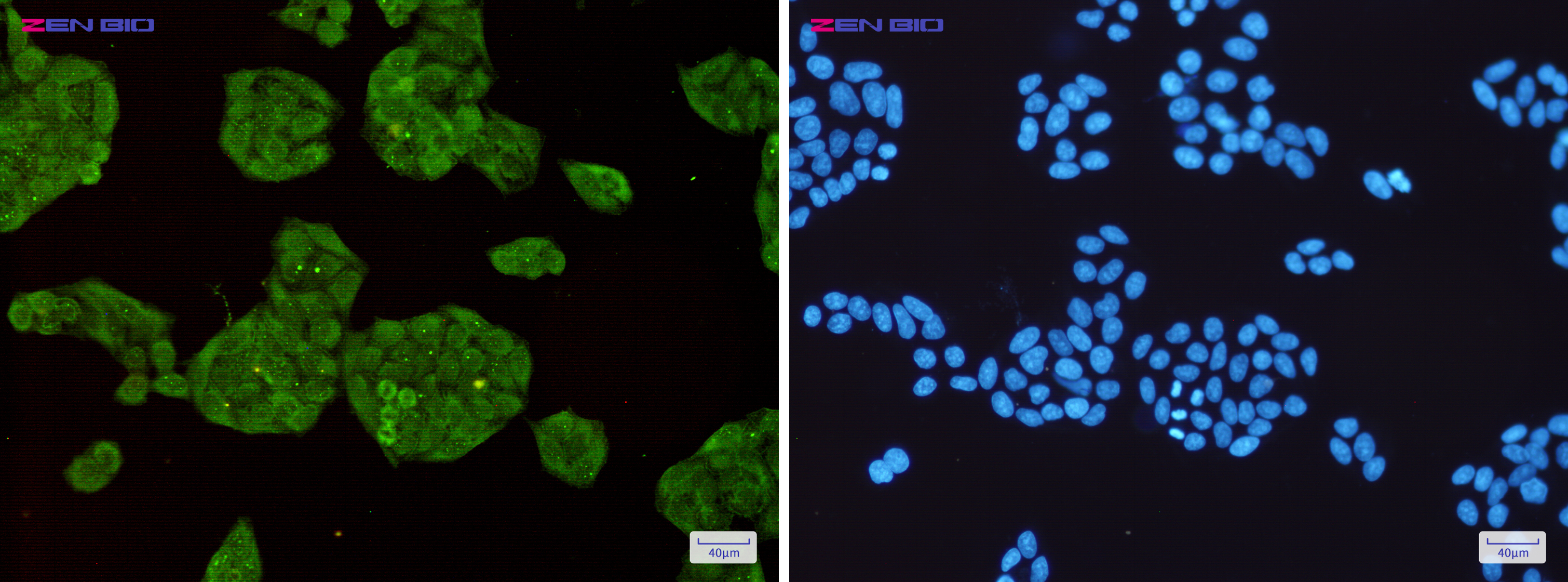
Immunocytochemistry of STUB1(green) in Hela cells using STUB1 Rabbit mAb at dilution 1/50, and DAPI(blue)
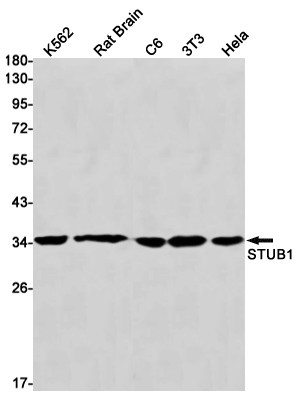
Western blot detection of STUB1 in K562,Rat Brain,C6,3T3,Hela cell lysates using STUB1 Rabbit mAb(1:1000 diluted).Predicted band size:35kDa.Observed band size:35kDa.
-
Background
Swiss-Prot Acc.Q9UNE7.E3 ubiquitin-protein ligase which targets misfolded chaperone substrates towards proteasomal degradation. Collaborates with ATXN3 in the degradation of misfolded chaperone substrates: ATXN3 restricting the length of ubiquitin chain attached to STUB1/CHIP substrates and preventing further chain extension. Ubiquitinates NOS1 in concert with Hsp70 and Hsp40. Modulates the activity of several chaperone complexes, including Hsp70, Hsc70 and Hsp90. Mediates transfer of non-canonical short ubiquitin chains to HSPA8 that have no effect on HSPA8 degradation. Mediates polyubiquitination of DNA polymerase beta (POLB) at 'Lys-41', 'Lys-61' and 'Lys-81', thereby playing a role in base-excision repair: catalyzes polyubiquitination by amplifying the HUWE1/ARF-BP1-dependent monoubiquitination and leading to POLB-degradation by the proteasome. Mediates polyubiquitination of CYP3A4. Ubiquitinates EPHA2 and may regulate the receptor stability and activity through proteasomal degradation. Acts as a co-chaperone for HSPA1A and HSPA1B chaperone proteins and promotes ubiquitin-mediated protein degradation (PubMed:27708256). Negatively regulates the suppressive function of regulatory T-cells (Treg) during inflammation by mediating the ubiquitination and degradation of FOXP3 in a HSPA1A/B-dependent manner (PubMed:23973223). Likely mediates polyubiquitination and downregulates plasma membrane expression of PD-L1/CD274, an immune inhibitory ligand critical for immune tolerance to self and antitumor immunity. Negatively regulates TGF-beta signaling by modulating the basal level of SMAD3 via ubiquitin-mediated degradation (PubMed:24613385). May regulate myosin assembly in striated muscles together with UBE4B and VCP/p97 by targeting myosin chaperone UNC45B for proteasomal degradation (PubMed:17369820). Mediates ubiquitination of RIPK3 leading to its subsequent proteasome-dependent degradation (PubMed:29883609).
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
