1. DOTA
Original Name: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
Molecular Structure:

Metals for Chelation: 52Mn, 55Co, 64Cu, 66Ga, 86Y, 89Zr, 152Tb
Literature References: 64Cu, 68Ga, 111In, 177Lu
Redox Potential: -0.74 (irreversible)
Dissociation Half-Life:<3 min (5 M HCl, 90℃)
Advantages: Multifunctionality. Its macrocyclic nature makes it a highly stable and easily modifiable chelator. Capable of forming complexes with various metal ions; easy to modify for different disease states. It is the only metal chelator that significantly impacts all major imaging modalities.
Applications: Ideal macrocyclic chelator for lanthanides but can also be used for other metals, mainly transition metals. Due to the size of the metal and the aperture of the macrocycle, 64Cu and 68Ga are the most documented DOTA ligands. DOTA can chelate 64Cu and 68Ga for PET scanning, and 111In and 90Y for SPECT and radiotherapy. It can be combined with paramagnetic lanthanide ions like gadolinium for MRI. Most octreotide analogs are labeled using DOTA chelators.
Limitations: In terms of coordination number, DOTA may not be the ideal chelator for 64Cu or 68Ga.
2. NOTA
Original Name: N,N′,N″-(1,4,7-triazacyclononane-1,4,7-triyl)triacetic acid
Molecular Structure:
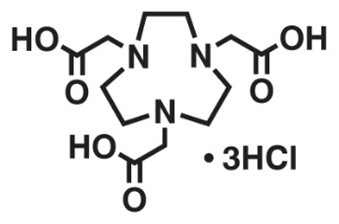
Metals for Chelation: 18F, 55Co, 64Cu, 66Ga, 68Ga
Redox Potential: -0.70 (irreversible)
Dissociation Half-Life:<3 min (5 M HCl, 90℃)
Advantages: NOTA, as a chelating agent, can form stable complexes with metal ions, thereby reducing the interference of metal ions with biomolecules. Composed of four nitrogen atoms, it forms a six-membered ring structure, capable of forming stable five-membered ring complexes with metal ions. The advantage of NOTA lies in its strong affinity for metal ions, stability of its complexes, and its non-toxicity and harmlessness to biomolecules.
Applications: NOTA is a bifunctional chelating agent, used in PET imaging, and also applied in probe design and signal amplification through multivalent effects.
3. TETA
Original Name: 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid
Molecular Structure:
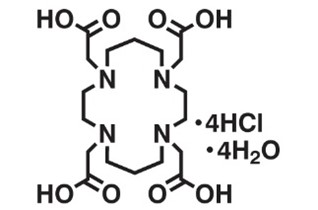
Metals for Chelation (according to the table): Ga, 55Co, 64Cu
Literature References: 64Cu
Applications: Currently, the preferred chelating agent for labeling biomolecules with copper radionuclides is an analog of TETA. For 64Cu chelate complexes, the 64Cu-TETA complex is more stable than the 64Cu-DOTA.
Redox Potential: -0.98 (irreversible)
Dissociation Half-Life:<3 min (5 M HCl, 90℃)
4. PCTA
Original Name: 3,6,9,15-tetraazabicyclo[9.3.1]pentadeca-1(15),11,13-triene-3,6,9-triacetic acid
Molecular Structure:
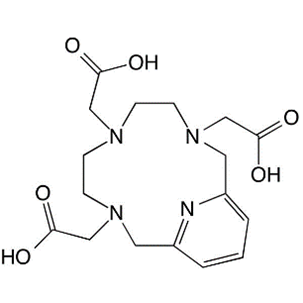
Metals for Chelation: 68Ga, 64Cu
Applications: Many bifunctional variants of PCTA have been synthesized and successfully applied. For example, targeting αvβ3 integrin for research using p-SCN-Bn-PCTA radiolabeled with 68Ga; chelating 90Y and 177Lu for radiotherapy; combining phospholipid biomolecule 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DSPE) with PCTA having additional carboxylic functionality (PCTA-COOH), radiolabeling this complex with 68Ga, and inserting it into high-density lipoprotein (HDL) particles (68Ga-PCTA-DSPE-HDL) for observation of atherosclerotic plaques.
5. DTPA
Original Name: N,N-bis[2-[bis(carboxymethyl)amino]ethyl]-glycine, Diethylenetriaminepentaacetic acid
Molecular Structure:
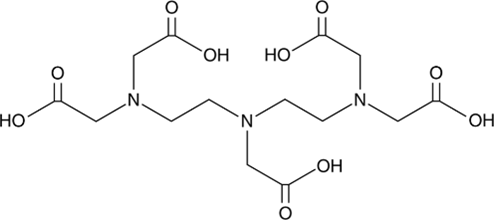
Metals for Chelation: Ga, Cu, Zr, 86Y
Literature References: Gd, Zn, 99Tc, Gd, Ca, 161Tb
Applications: A non-cyclic chelator. Since the 1960s, DTPA has been used by doctors to treat contamination caused by radioactive substances entering the human body through respiration, diet, or open wounds. The FDA has only approved DTPA for chelating three radioactive substances: Pu (plutonium), Am (americium), and Cm (curium). Both forms of DTPA, calcium (Ca-DTPA) and zinc (Zn-DTPA), can tightly chelate plutonium, americium, and curium. Radioactive substances are difficult to remove from the body after 24 hours of entry, but DTPA can still clear these substances from the body days or even weeks after contamination.
Commonly Used Radioactive Nuclides in Nuclear Labeling
1. SPECT Imaging: 99Tc (6.0h), 131I (8.04d), 133Xe (5.25d), 67Ga (3.26d), 111In (2.80d), 81Kr, 123I (13.2h), 201Tl
2. PET Imaging: 18F (1.8h), 68Ga (1.1h), 64Cu (12.7h), 11C, 13N, 15O
3. Radioimmunoassay: 125I (60.1d), 3H (12.32a)
4. Therapeutic Radiopharmaceuticals: 131I (8.04d), 32P (14.3d), 90Y (2.67d), 169Er (9.42d), 153Sm, 117Sn, 166Ho (26.8h), 177Lu (6.71d), 188Re (17.0h), 223Ra (11.4d), 225Ac (10.0d), 227Th (19.0d), 211At (7.2h)
5. External Beam Radiotherapy: 60Co
6. Brachytherapy: 192Ir, 125I (60.1d), 137Cs, 198Au (2.7d), 106Ru, 103Pd (17.1d)
Souce: NovoPro 2024-01-26
